I hope that everyone had a safe holiday week! I’m putting these updates into a digest form so you can see what happened last week regarding COVID information.
Updated Strategies for Optimizing the Supply of Facemasks (CDC)— This guidance has been updated to include the following:
- Added considerations for returning to conventional capacity practices
- Conventional Capacity Strategies
- Clarified healthcare personnel (HCP) use of facemasks for source control and as PPE to protect their nose and mouth from exposure to splashes, sprays, and respiratorysecretions
- Contingency Capacity Strategies
- Added clarifications on extended use of facemasks as PPE
- Added clarifications on restriction on facemask use by HCP as PPE rather than by patients for source control
- Crisis Capacity Strategies
- Revised section on limited re-use paired with extended use
- Deleted the strategies of designating convalescent HCP for the provision of care of patients with SARS-CoV-2 infection, use of expedient patient isolation rooms, and mechanical headboards
Scientific Brief: Community Use of Cloth Masks to Control the Spread of SARS-CoV-2 (CDC) — CDC has issued a brief that reviews and analyzes the rapidly growing body of science supporting use of cloth masks in communities to control the spread of COVID-19.
Long-Term Care Staffing During COVID-19 (IDPH) – a 3-page document - was issued to Iowa LTCFs “in response to a growing number of facilities experiencing challenges in staffing normal operations during the pandemic, the state has worked to create a workflow that can be leveraged by facilities to more effectively engage partners that can assist during an event causing a staffing shortage.”
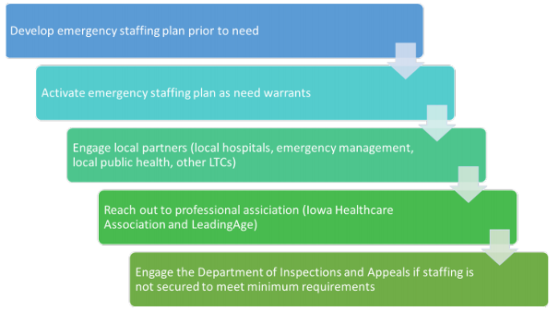
You’ll note this on the bottom of page 2:

Resources and contact information is found on page 3.
Face Masks, Including Surgical Masks, and Respirators for COVID-19 (FDA)provides information on FDA-regulated face masks (including cloth face coverings), surgical masks, and respirators (filtering facepiece respirators, such as N95 respirators) intended for a medical purpose to assist in preventing the spread of infectious materials during the COVID-19 pandemic.

COVID-19 Vaccines (CDC) shows an update of November 25, 2020 but I’m not able to see what was specifically updated. I did spot some updated information on the ACIP Meeting Information link as well as some of the other links on the vaccines page. Additional info on COVID-19 vaccines is also found on this Vaccination FAQ website.
State COVID-19 Vaccination Plans are available for some specific states:
- Texas
- Nebraska
- Arkansas
- Mississippi
- Puerto Rico
- U.S. Virgin Islands
NHSN LTCF Module – new Pathway forms, templates and instructions.
Resident Impact and Facility Capacity:
- COVID-19 Resident Impact and Facility Capacity Pathway Form (November 23, 2020)
- Instructions for Completion of COVID-19 LTCF Resident Impact and Facility Capacity Form (November 23, 2020)
Staff and Personnel Impact:
- Long Term Care Facility: Staff and Personnel Impact Form (November 23, 2020)
- Instructions for Completion of COVID-19 LTCF Staff and Personnel Impact Form (November 23, 2020)
Facility Resources:
- How to Upload COVID-19 CSV Data Files (November 23, 2020)
- Resident Impact and Facility Capacity Template (Excel) (November 23, 2020)
- Staff and Personnel Impact Template (Excel) (November 23, 2020)
Group Resources:
- Guidance for use of COVID-19 Module Data by NHSN Health Department Groups (November 20, 2020)
- Groups and Supergroups – Viewing and Uploading COVID-19 CSV Data Files (November 23, 2020)
- Resident Impact and Facility Capacity Template (Excel) (November 23, 2020)
- Staff and Personnel Impact Template (Excel) (November 23, 2020)
Want to keep up with the changing COVID-19 situation in skilled nursing?




