Welcome to 2021! I hope that you, your families and your facility/community/agency families were able to enjoy a safe and wonderful holiday season these past 2 weeks. Let’s catch up on what’s happened during Christmas and New Years’ weeks:
Upcoming Webinars:
- Vaccination in Long Term Care: Safety, Efficacy, and Residents' Rights is scheduled for January 12, 2021. Research scientist Beth Nivin, MPH, will lead a discussion on the safety and efficacy of the COVID and influenza vaccines in long-term care settings. This 1-hour webinar is sponsored by the Long Term Care Community Coalition.
HHS/CMS/U.S. Senate Correspondence:
- Click here to view the letter sent to HHS and CMS by Senators Wyden and Casey (ranking members of the Senate Finance and Aging Committees, respectively) on December 21, 2020. It’s an interesting and quick read that makes some excellent points:
- More than 115,000 residents and workers of long-term care facilities in the United States have died of COVID-19 to date, and Federal data show that the pace of COVID19 infections and deaths in nursing homes is rising rapidly. In the last two weeks alone, roughly 11,000 residents and workers in long-term care facilities succumbed to COVID-19, underscoring the pressing need to swiftly and efficiently administer vaccines in these settings.
- Ensure that long-term care workers have ready access to the vaccine – this will be critical to stopping the COVID-19 crisis in our nation’s nursing homes. (Be
- Immediately make public the data provided to the Federal government by CVS and Walgreens and begin collecting and posting facility-level data about the number of residents and workers in nursing homes vaccinated outside of the LTC Partnership. By year’s end, nursing homes should begin reporting the number of residents and workers who have received COVID-19 vaccines; these data should, in turn, be included in the weekly COVID-19 reports issued by CMS.
- COVID-19 vaccination data should be included in Minimum Data Set reporting requirements and Nursing Home Compare—similar to flu and pneumonia vaccines.
Toolkits:
- AHCA/NCAL has provided a Communications Toolkit to “encourage long term care staff and residents to get the COVID-19 vaccine. Providers and state affiliates may use the materials below to help communicate about the importance of vaccination as well as highlight progress. This digital toolkit includes a checklist of ideas, template letters to use with stakeholders, sample social media, and media prep material. Keep checking back for additional resources!” Here’s what you’ll see on that website:
- Toolkit for Healthcare Providers was updated December 29, 2020.
- Toolkit on State Actions to Mitigate COVID-19 Prevalence in Nursing Homes ... Version 16is now available.
CDC Updates:
- 8 Things to Know about the U.S. COVID-19 Vaccination Program was updated on December 22, 2020. COVID-19 Vaccination ... Clinical Resources for Each COVID-19 Vaccine was updated on December 20, 2020 as was U.S. COVID-19 Vaccine Product Information. Beneath the product information website, you’ll find a wealth of information:
- Immunization Courses: Webcasts and Self Study is a website with great resources regarding immunization, some offering CEU credits as well as certificates of training. Here’s just a few of the courses available:
- Quick Guide to Information on the New COVID-19 Vaccinations was updated by CDC and CMS on December 30, 2020.
- Importance of COVID-19 Vaccination for Residents of Long-term Care Facilities was updated December 28, 2020.
Other:
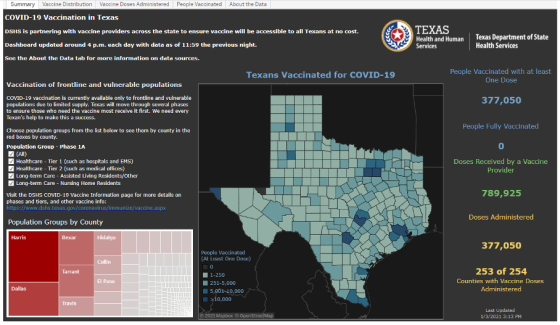
- Texas has launched an online COVID-19 vaccine tracking tool that shows vaccine administration information by county. The dashboard will be updated as new information becomes available.
- COVID-19 Vaccine Codes: Updated Effective Date for Moderna is a special edition of MLN Connects issued December 22, 2020.
On December 18, 2020, the U.S. Food and Drug Administration issued an Emergency Use Authorization (EUA) for the Moderna COVID‑19 Vaccine (PDF) for the prevention of COVID-19 for individuals 18 years of age and older. Review Moderna’s Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) regarding the limitations of authorized use.
During the COVID-19 Public Health Emergency (PHE), Medicare will cover and pay for the administration of the vaccine (when furnished consistent with the EUA). Review our updated payment and HCPCS Level I CPT code structure for specific COVID-19 vaccine information. Only bill for the vaccine administration codes when you submit claims to Medicare; don’t include the vaccine product codes when the vaccines are free
Related links:
- CMS COVID-19 Provider Toolkit
- FDA COVID-19 Vaccines webpage
- COVID-19 Vaccine Codes: Updated Effective Date for Pfizer-BioNTech is a special edition of MLN Connects issued December 14, 2020.
On December 11, 2020, the U.S. Food and Drug Administration issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech COVID‑19 Vaccine for the prevention of COVID-19 for individuals 16 years of age and older. Review Pfizer’s Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) regarding the limitations of authorized use.
During the COVID-19 Public Health Emergency (PHE), Medicare will cover and pay for the administration of the vaccine (when furnished consistent with the EUA). Review our updated payment and HCPCS Level I CPT code structure for specific COVID-19 vaccine information. Only bill for the vaccine administration codes when you submit claims to Medicare; don’t include the vaccine product codes when vaccines are free.
Related links:
- CMS COVID-19 Provider Toolkit
- CMS COVID-19 FAQs (PDF)
- FDA COVID-19 Vaccines webpage
Want to keep up with the changing COVID-19 situation in skilled nursing?




